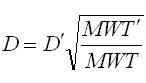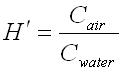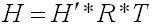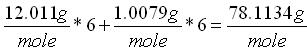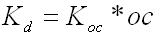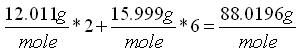Copyright © 2023 ESCI, LLC - All Rights Reserved.
SESOIL Chemical Input Parameters
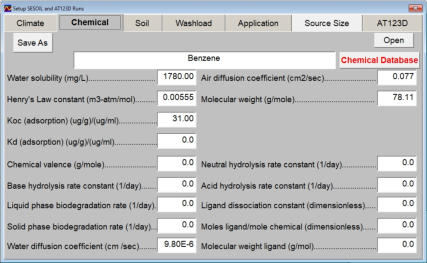
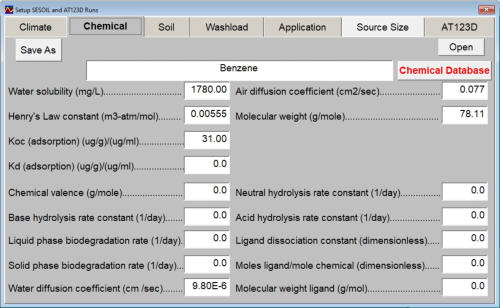
The chemical input tab contains information on physical properties of the contaminants. These properties are used by the SESOIL pollutant cycle sub-model to predict the transport and fate of contaminants in the soil column. The large number of chemical input parameters means that SESOIL can be used for a wide range of contaminant types. I have divided these parameters in two categories based on use. Commonly used chemical parameters: • Water solubility, • Air and water diffusion coefficients, • Henry′s Law Constant, • Organic carbon adsorption coefficient (K oc ), and • Soil partition coefficient (K d ). Rarely used parameters: • Molecular weight, • Valence, • Biodegradation, • Hydrolysis rate constants, and • Ligand properties. As SEVIEW contains a chemical database for the most part all you need to do is find your chemical. Properties for additional contaminants can be found on the internet or in various references. A detailed description of each input parameter is provided below. Additional information on these parameters can be found in the SEVIEW help file and User's Guide. Commonly Used Parameters These parameters are typically used when performing SESOIL modeling. Water Solubility Water solubility is the maximum aqueous concentration a substance can reach in mg/ml. This property is temperature dependent and is typically measured at 25 o C. Diffusion Coefficients Diffusion consists of the random motion of atoms or molecules in a gas or liquid. This motion causes contaminants to disperse from areas of higher concentration to areas of lower concentration. Diffusion is controlled by molecular weight, in that lighter atoms or molecules are dispersed at higher rates. This dependency on weight can be used to establish diffusion coefficients for additional chemicals using the equation below: Where D =Diffusion coefficient of the current molecule in cm 2 /sec D’ = Diffusion coefficient for a reference molecule in cm 2 /sec MWT′ = Molecular weight of the reference molecule in g/mole MWT = Molecular weight of the current molecule in g/mole This equation can be used to estimate both air and water diffusion coefficients. Please note that almost any substance can be used as the reference molecule. Air Diffusion Coefficient The air diffusion coefficient for a chemical in cm 2 /sec. Water Diffusion Coefficient The water diffusion coefficient for a chemical in cm 2 /sec. This parameter is not used by SESOIL. It was added to SEVIEW to simplify the link between SESOIL and AT123D. Addition of this parameter means that users no longer need to edit the water diffusion coefficient in AT123D prior to running the model. Henry’s Law Constant The Henry′s Law constant is the air:water partitioning coefficient in m 3 -atm/mol. It relates the chemical concentrations between the gaseous and aqueous phases. The dimensionless form is simply the ratio of the chemical concentration in air to that in water. Where C air = Concentration in air in µg/L C water = Concentration in water in µg/L H′ = Dimensionless Henry′s Law constant SESOIL uses the dimensional form of the Henry′s constant in m 3 -atm/mol. The dimensionless form can be converted to the dimensional form using the equation below: Where H = Henry′s Law constant in m 3 -atm/mol H′ = Dimensionless Henry′s Law constant in (µ/L)/(µ/L) R = Universal gas constant, in m3-atm/mol-K = 8.2 E-5, and T = Temperature K = 298 Organic Carbon Adsorption Coefficient The organic carbon adsorption coefficient (K oc ) is the chemical adsorption coefficient normalized to the organic carbon content of the soil in (µg/g)/(µg/ml). This parameter establishes the tendency of a substance to bind to organic matter in the soil. SESOIL uses K oc and the soil organic carbon content to calculate the soil partition coefficient (K d ). Where K d = Soil partition coefficient in (µg/g)/(µg/ml) K oc = Organic carbon adsorption coefficient in (µg/g)/(µg/ml) oc = Fraction soil organic carbon content Molecular Weight Molecular weight is the sum of the atomic weights of the atoms. For example, the molecular weight of benzene (C 6 H 6 ) which consists of six carbon and six hydrogen atoms is calculated below: The atomic weights for carbon and hydrogen are 12.011 g/mole and 1.0079 g/mole respectively. SESOIL only uses molecular weight if the complexation or cation exchange algorithms are used. Biodegradation Biodegradation is the process by which a chemical is converted to a new form via biotic reactions. SESOIL simulates biodegradation as a first-order decay process. If used values are generally based on site-specific measurements or extremely low generic values. Liquid Phase Biodegradation Liquid phase biodegradation occurs to contaminants contained in soil moisture within the vadose zone. In general, the liquid phase biodegradation rate constant is higher than in the solid phase. Solid Phase Biodegradation Solid phase biodegradation occurs to contaminants adsorbed on soil within the vadose zone. Valence of the Chemical The positive valence charge for a substance. Valence is controlled by the number of electrons in the outermost orbital of an atom. Metals typically loose one or more valence electron creating positive ions. These valence electrons control the type of chemical bonds and thus formation of compounds. SESOIL uses valence along with the soil cation exchange capacity to simulate the sorption of metals to soil. Hydrolysis Rate Constants Hydrolysis is a chemical reaction between salts of weak acids and bases with water. The presence of these salts causes the water to ionize into negative hydroxyl ions (OH - ) and positive hydrogen ions (H + ). This results in the formation of neutral, basic, or acidic solutions. Exactly which type of solution is formed depends on the properties of the salt ions. Neutral The neutral hydrolysis rate constant (L/mol/day). Base The base hydrolysis rate constant (L/mol/day). Acid The acid hydrolysis rate constant (L/mol/day). Ligand Properties Ligands are groups of molecules that attach to metal ions. Ligands (also known as coordinating groups) basically take the place of an anion in a cation-anion molecule. Such cation-ligand substances are called a complex. Ligand Stability Constant The ligand stability constant is the dimensionless ratio that relates the tendency of a complex to dissociate. The more stable the complex the higher the ligand stability constant. Moles Ligand per Mole Compound The dimensionless ratio of the moles of ligand to the moles of the compound. Molecular Weight of the Ligand Like molecular weight the weight of the ligand is the sum of the atomic weights of the atoms in g/mole. For example, molecular weight of the ligand oxalato (C 2 O 4 2- ) consists of two carbon and four oxygen atoms: The atomic weights for carbon and oxygen are 12.011 g/mole and 15.9994 g/mole respectively.
SEVIEW
Transport and fate modeling software
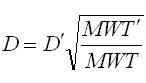
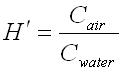
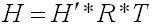
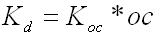
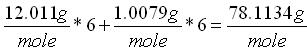
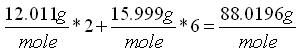

SESOIL Chemical Input
Parameters
The chemical input tab contains information on physical properties of the contaminants. These properties are used by the SESOIL pollutant cycle sub-model to predict the transport and fate of contaminants in the soil column. The large number of chemical input parameters means that SESOIL can be used for a wide range of contaminant types. I have divided these parameters in two categories based on use. Commonly used chemical parameters: • Water solubility, • Air and water diffusion coefficients, • Henry′s Law Constant, • Organic carbon adsorption coefficient (K oc ), and • Soil partition coefficient (K d ). Rarely used parameters: • Molecular weight, • Valence, • Biodegradation, • Hydrolysis rate constants, and • Ligand properties. As SEVIEW contains a chemical database for the most part all you need to do is find your chemical. Properties for additional contaminants can be found on the internet or in various references. A detailed description of each input parameter is provided below. Additional information on these parameters can be found in the SEVIEW help file and User's Guide. Commonly Used Parameters These parameters are typically used when performing SESOIL modeling. Water Solubility Water solubility is the maximum aqueous concentration a substance can reach in mg/ml. This property is temperature dependent and is typically measured at 25 o C. Diffusion Coefficients Diffusion consists of the random motion of atoms or molecules in a gas or liquid. This motion causes contaminants to disperse from areas of higher concentration to areas of lower concentration. Diffusion is controlled by molecular weight, in that lighter atoms or molecules are dispersed at higher rates. This dependency on weight can be used to establish diffusion coefficients for additional chemicals using the equation below: Where D =Diffusion coefficient of the current molecule in cm 2 /sec D’ = Diffusion coefficient for a reference molecule in cm 2 /sec MWT′ = Molecular weight of the reference molecule in g/mole MWT = Molecular weight of the current molecule in g/mole This equation can be used to estimate both air and water diffusion coefficients. Please note that almost any substance can be used as the reference molecule. Air Diffusion Coefficient The air diffusion coefficient for a chemical in cm 2 /sec. Water Diffusion Coefficient The water diffusion coefficient for a chemical in cm 2 /sec. This parameter is not used by SESOIL. It was added to SEVIEW to simplify the link between SESOIL and AT123D. Addition of this parameter means that users no longer need to edit the water diffusion coefficient in AT123D prior to running the model. Henry’s Law Constant The Henry′s Law constant is the air:water partitioning coefficient in m 3 -atm/mol. It relates the chemical concentrations between the gaseous and aqueous phases. The dimensionless form is simply the ratio of the chemical concentration in air to that in water. Where C air = Concentration in air in µg/L C water = Concentration in water in µg/L H′ = Dimensionless Henry′s Law constant SESOIL uses the dimensional form of the Henry′s constant in m 3 -atm/mol. The dimensionless form can be converted to the dimensional form using the equation below: Where H = Henry′s Law constant in m 3 -atm/mol H′ = Dimensionless Henry′s Law constant in (µ/L)/(µ/L) R = Universal gas constant, in m3-atm/mol-K = 8.2 E-5, and T = Temperature K = 298 Organic Carbon Adsorption Coefficient The organic carbon adsorption coefficient (K oc ) is the chemical adsorption coefficient normalized to the organic carbon content of the soil in (µg/g)/(µg/ml). This parameter establishes the tendency of a substance to bind to organic matter in the soil. SESOIL uses K oc and the soil organic carbon content to calculate the soil partition coefficient (K d ). Where K d = Soil partition coefficient in (µg/g)/(µg/ml) K oc = Organic carbon adsorption coefficient in (µg/g)/(µg/ml) oc = Fraction soil organic carbon content Molecular Weight Molecular weight is the sum of the atomic weights of the atoms. For example, the molecular weight of benzene (C 6 H 6 ) which consists of six carbon and six hydrogen atoms is calculated below: The atomic weights for carbon and hydrogen are 12.011 g/mole and 1.0079 g/mole respectively. SESOIL only uses molecular weight if the complexation or cation exchange algorithms are used. Biodegradation Biodegradation is the process by which a chemical is converted to a new form via biotic reactions. SESOIL simulates biodegradation as a first-order decay process. If used values are generally based on site-specific measurements or extremely low generic values. Liquid Phase Biodegradation Liquid phase biodegradation occurs to contaminants contained in soil moisture within the vadose zone. In general, the liquid phase biodegradation rate constant is higher than in the solid phase. Solid Phase Biodegradation Solid phase biodegradation occurs to contaminants adsorbed on soil within the vadose zone. Valence of the Chemical The positive valence charge for a substance. Valence is controlled by the number of electrons in the outermost orbital of an atom. Metals typically loose one or more valence electron creating positive ions. These valence electrons control the type of chemical bonds and thus formation of compounds. SESOIL uses valence along with the soil cation exchange capacity to simulate the sorption of metals to soil. Hydrolysis Rate Constants Hydrolysis is a chemical reaction between salts of weak acids and bases with water. The presence of these salts causes the water to ionize into negative hydroxyl ions (OH - ) and positive hydrogen ions (H + ). This results in the formation of neutral, basic, or acidic solutions. Exactly which type of solution is formed depends on the properties of the salt ions. Neutral The neutral hydrolysis rate constant (L/mol/day). Base The base hydrolysis rate constant (L/mol/day). Acid The acid hydrolysis rate constant (L/mol/day). Ligand Properties Ligands are groups of molecules that attach to metal ions. Ligands (also known as coordinating groups) basically take the place of an anion in a cation-anion molecule. Such cation-ligand substances are called a complex. Ligand Stability Constant The ligand stability constant is the dimensionless ratio that relates the tendency of a complex to dissociate. The more stable the complex the higher the ligand stability constant. Moles Ligand per Mole Compound The dimensionless ratio of the moles of ligand to the moles of the compound. Molecular Weight of the Ligand Like molecular weight the weight of the ligand is the sum of the atomic weights of the atoms in g/mole. For example, molecular weight of the ligand oxalato (C 2 O 4 2- ) consists of two carbon and four oxygen atoms: The atomic weights for carbon and oxygen are 12.011 g/mole and 15.9994 g/mole respectively.
SEVIEW
Transport and fate modeling software